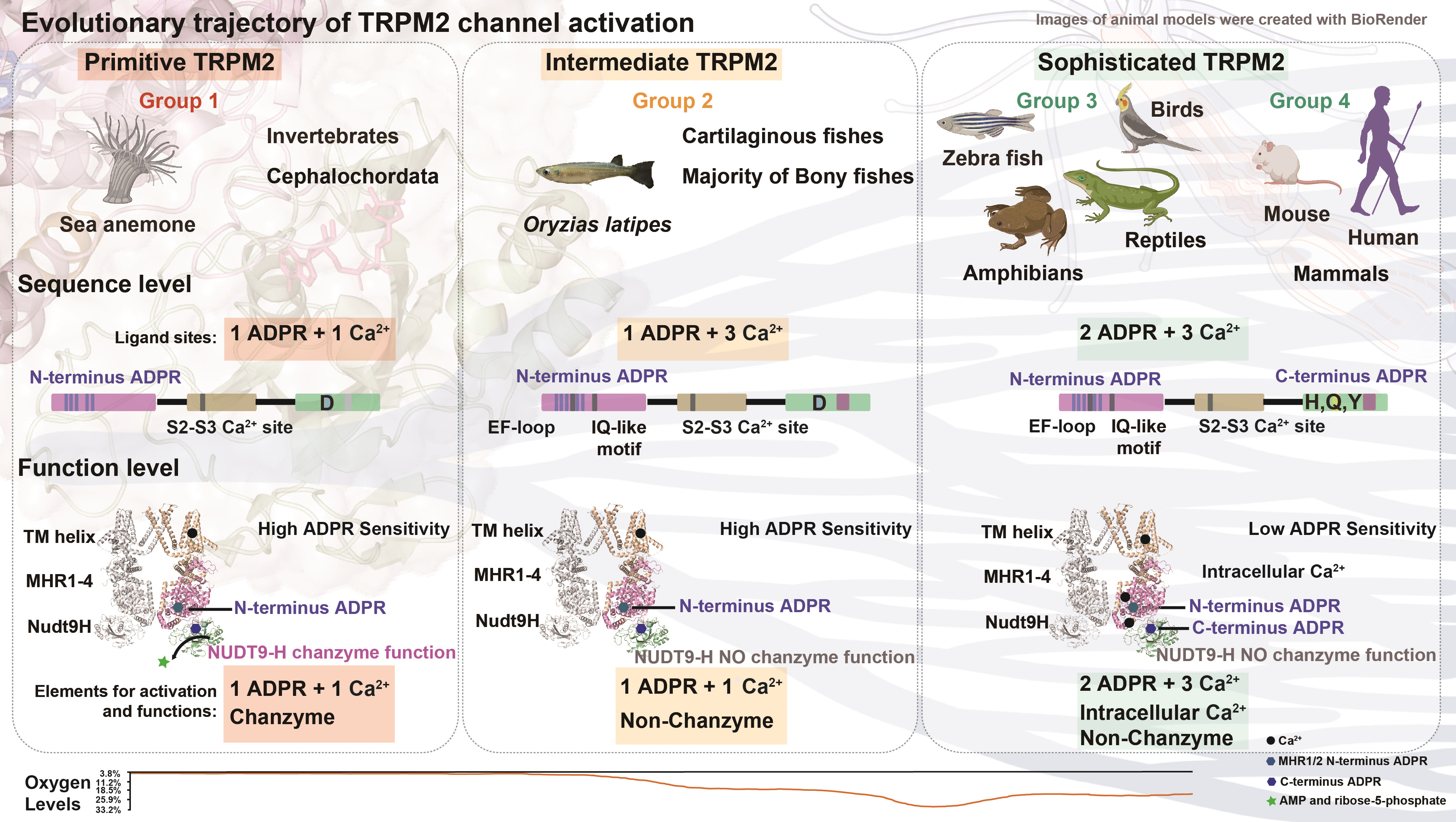
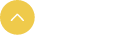Angew. Chem. Int. Ed.| Zhejiang University Tang Longguang’s team developed novel nanodrugs for cancer theranostics
Recently, the research team led by Prof. Longguang Tang from International Institutes of Medicine, The Fourth Affiliated Hospital of Zhejiang University, together with Prof. Xiaoyuan Chen from National University of Singapore, and Prof. Zijian Zhou from Xiamen University published an article entitled ‘Coordinating the mechanism of actions of ferroptosis and photothermal effect for cancer theranostics’ in one of the top-rank journal of Chemistry: Angewandte Chemie International Edition. The paper proposed and constructed a theranostic nano-platform based on ‘reciprocal’ coordinating enhance effect which is expected to provide fresh ideas for developing novel antineoplastic drugs.

The traditional strategy using the combination of two or more anti-tumor modes is often difficult to optimize the efficacy of cancer therapy due to the lack of consideration of the internal mechanism correlation between different modes. The author constructed a nano theranostic platform based on croconaine dye from the internal mechanism of photothermal therapy and ferroptosis (Cro-Fe@BSA) where the synergistic effect of combined therapy between different anti-tumor modes was considered.
The croconaine dye molecules encapsulated in BSA acts as a ferric ion chelating agent and photothermal conversion agent which activates the photothermal effect in the tumor micro-environment. Meanwhile, the coordinated ferric iron is converted into active divalent iron under the reduction of glutathione, triggering Fenton reaction. This process not only enhances the accumulation of intracellular active iron pool (divalent iron), but also consumes the antioxidant reducing protein (GPX4), eventually resulting in cell iron death.
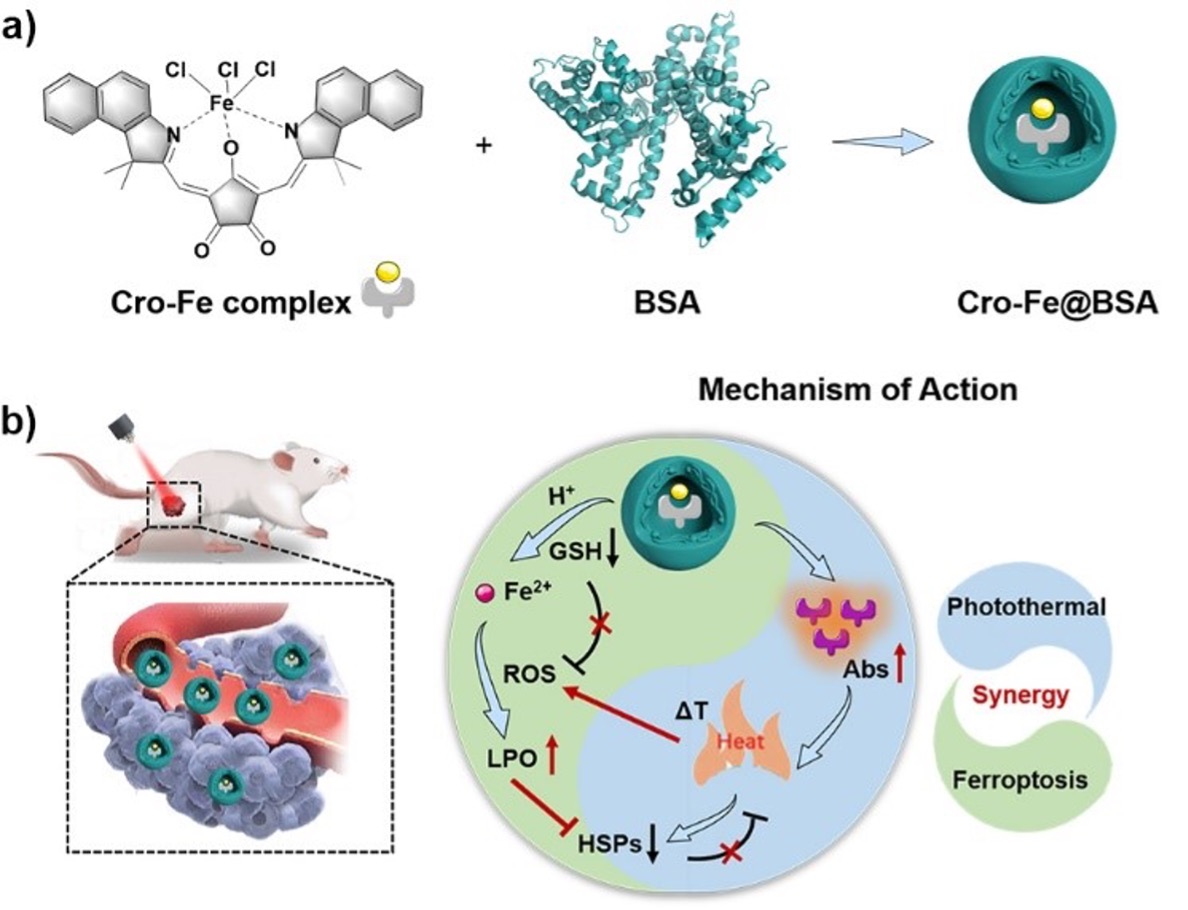
Figure 1 The theranostic nano platform based on ‘reciprocal’ coordinating enhance effect. (a) Fabrication of Cro-Fe@BSA nanoparticles by the encapsulation of iron (III)-coordinated croconaine molecules with bovin serum albumin. (b) Mechanism of the mutually beneficial combination of ferroptosis and the photothermal effect for cancer therapy.
It is interesting to note that the photothermal effect produced by nanomaterials not only acts as the photothermal therapy, but also drives the Fenton reaction process and enhance the tumor killing ability of ferroptosis. Additionally, the highly active hydroxy radicals and lipid peroxides accumulated in cells inhibit the expression of photothermal resistance protein (HSP70) thereby weakens photothermal resistance ability of tumor cells. At the same time, the in vivo photoacoustic and magnetic resonance imaging contrast performance provides molecular imaging tracking and verification for tumor treatment. This theranostic nano-platform based on the "reciprocal" synergistic enhancement mechanism illustrated superior tumor inhibition effect in the mouse breast cancer tumor model, providing a new idea for developing novel anti-tumor drugs for diagnosis and treatment.
Figure 2. (a) The proposed mechanism of the mutually beneficial synergistic effect of PTT and ferroptosis. (b) The cell viability profiles of 4T1 cells after incubation with different concentrations of Cro-Fe@BSA under laser irradiation with different power densities (0.5 and 1.0 W/cm2). (c, d) The GSH and the ferrous ions in 4T1 cells incubated with different treatments, respectively (n = 3/group). (e) The confocal fluorescence images of 4T1 cells after incubation with different treatments. The BODIPY (Ox) indicates the oxidized form of C11-BODIPY581/591. Groups I-VI represent control, Cro@BSA, Cro@BSA + laser,Cro-Fe@BSA, Cro-Fe@BSA + laser, and Cro-Fe@BSA + Fer-1, respectively. Fer-1 indicates a ferroptosis inhibitor Ferrostatin-1. (f, g) The GPX4 and HSP70 expression levels by western blot analysis. *p < 0.05; **p < 0.01; ***p < 0.001, ****p < 0.0001, analyzed by two-sided Student‘s t-test.
It is interesting to note that the photothermal effect produced by nanomaterials not only acts as the photothermal therapy, but also drives the Fenton reaction process and enhance the tumor killing ability of ferroptosis. Additionally, the highly active hydroxy radicals and lipid peroxides accumulated in cells inhibit the expression of photothermal resistance protein (HSP70) thereby weakens photothermal resistance ability of tumor cells. At the same time, the in vivo photoacoustic and magnetic resonance imaging contrast performance provides molecular imaging tracking and verification for tumor treatment. This theranostic nano-platform based on the "reciprocal" synergistic enhancement mechanism illustrated superior tumor inhibition effect in the mouse breast cancer tumor model, providing a new idea for developing novel anti-tumor drugs for diagnosis and treatment.