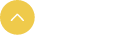Asthma is one of the most common chronic airway inflammatory diseases in the world, and its prevalence is increasing year by year. However, the clinical control of asthma, especially severe asthma, is still not ideal under the existing treatment strategies. On August 16, 2022, the team of professors Ying Songmin and Shen Huahao from Zhejiang University School of Medicine published the research results online entitled Treatment of allergic eosinophilic asthma through engineered IL-5-anchored chimeric antigen receptor T cells in "Cell Discovery" . In this study, the designed chimeric IL-5-CD28-CD3ζ receptor illustrated the framework for the cytokine-anchored chimeric antigen receptor (CCAR) system, revealing the IL-5-anchored CCAR-T (hereinafter referred to as CCAR-T) cells targeted killing of eosinophils and protective effects on allergic airway inflammation in vitro and in vivo, provided a potential new strategy for the treatment of allergic asthma. This study innovatively exploits the CCAR-T cell strategy for asthma treatment, which may be a milestone achievement in future research on allergic disease treatment.

Over 339 million people suffer from asthma worldwide and patients with severe eosinophilic asthma (SEA) are at high risk of mortality and low quality of life. SEA, characterized by eosinophilic inflammation, is a major phenotype of refractory asthma with poor clinical control. Eosinophilia is closely related to higher exacerbation frequency and worse control, leading to decreased lung function. Thus, strategies capable of inactivating or depleting eosinophils offer attractive therapies for SEA.
Currently, asthma symptoms control mainly relies on the daily administration of the inhaled corticosteroids (ICS) combined with β2 receptor agonists, but this therapy cannot completely relieve the asthma process. Novel biological agents targeting the interleukin-5 (IL-5)/IL-5 receptor α (IL-5Rα) axis interfere with the pathologic functions of eosinophils and show promising therapeutic effects for SEA patients. However, the therapeutic effect of monoclonal antibody drugs is still not ideal and requires long-term and repeated administration. Therefore, the development of novel long-lasting therapeutics targeting eosinophils is of great significance for the treatment of SEA.
Dr. Chen Sisi, the first author of the paper, said that this study designed a CCAR-T cell therapy strategy targeting eosinophils, and in vitro and in vivo experiments demonstrated that CCAR-T cells could effectively target eosinophils and continuously reduce their levels in a mouse model of chronic airway inflammation. Previous studies have shown that chronic airway inflammation is one of the most important clinicopathological feature of asthma. Our study found that CCAR-T cells can effectively inhibit the secretion of inflammatory factors and infiltration of inflammatory cells in the airway, and have a significant relieving effect on asthma.
The research team of this project has long been committed to the research of eosinophils in chronic airway inflammatory diseases such as asthma. In recent years, they have successively elucidated that eosinophils play an important role in the maintenance of hematopoietic stem cell homeostasis during the pathogenesis of asthma (Cell Res 2018), confirmed the promotion of tumor lung metastasis upon eosinophilic inflammation (Sci Adv 2021), and revealed the key role of the CCL6/CCR1 axis in promoting eosinophil differentiation and airway inflammation (Signal Transduct Target Ther 2021), analyzed the high-resolution electron microscope structure of CCR1 and other related key proteins (Nat Chem Biol 2022, Cell Discov 2022), and proposed targeting and subsequently inducing eosinophil apoptosis as a potential strategy in the treatment of hormone-resistant asthma (J Allergy Clin Immunol 2017, Biomaterials 2019).
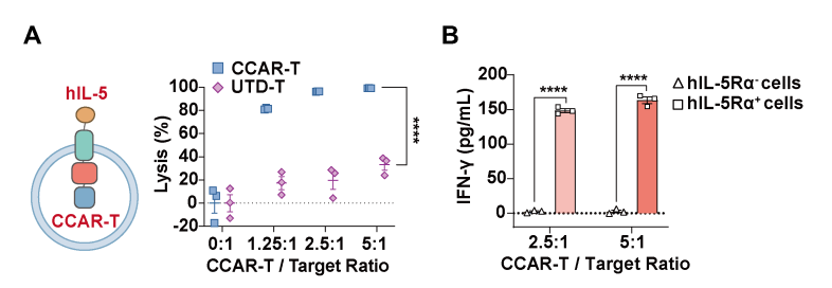
Figure 1. In vitro killing specificity and efficiency of CCAR-T cells
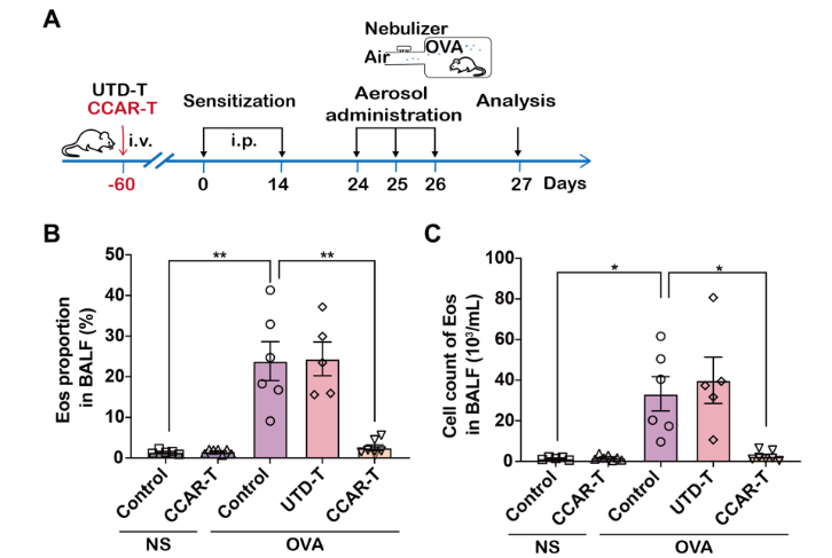
Figure 2. CCAR-T cells exhibit durable asthma control
The research was supported by the National Key R&D Program of China, National Natural Science Foundation of China, Zhejiang Provincial Natural Science Foundation, Medical Health Science and Technology Project of Zhejiang Provincial Health Commission and so on. Chen Sisi, Chen Gaoying and Xu Fang, doctoral candidates of Zhejiang University School of Medicine , are the co-first authors of the paper, and Professor Ying Songmin and Shen Huahao are the co-corresponding authors of the paper. This work has also received strong support and assistance from Professor Chen Wei and Professor Sun Jie of Zhejiang University School of Medicine.