A pioneering study led by Prof. Yang Wei from International School of Medicine, Zhejiang University, Dr. Ma Cheng, Luo Yanping, and Zhang Congyi from Zhejiang University School of Medicine has unveiled the intricate evolutionary journey of TRPM2 ion channels, offering insights into how these proteins have adapted over millions of years. The research, published in Science Bulletin, provides a comprehensive analysis of TRPM2 channels from over 280 species, shedding light on their evolutionary trajectory in channel activation modes by ADPR and Ca2+.
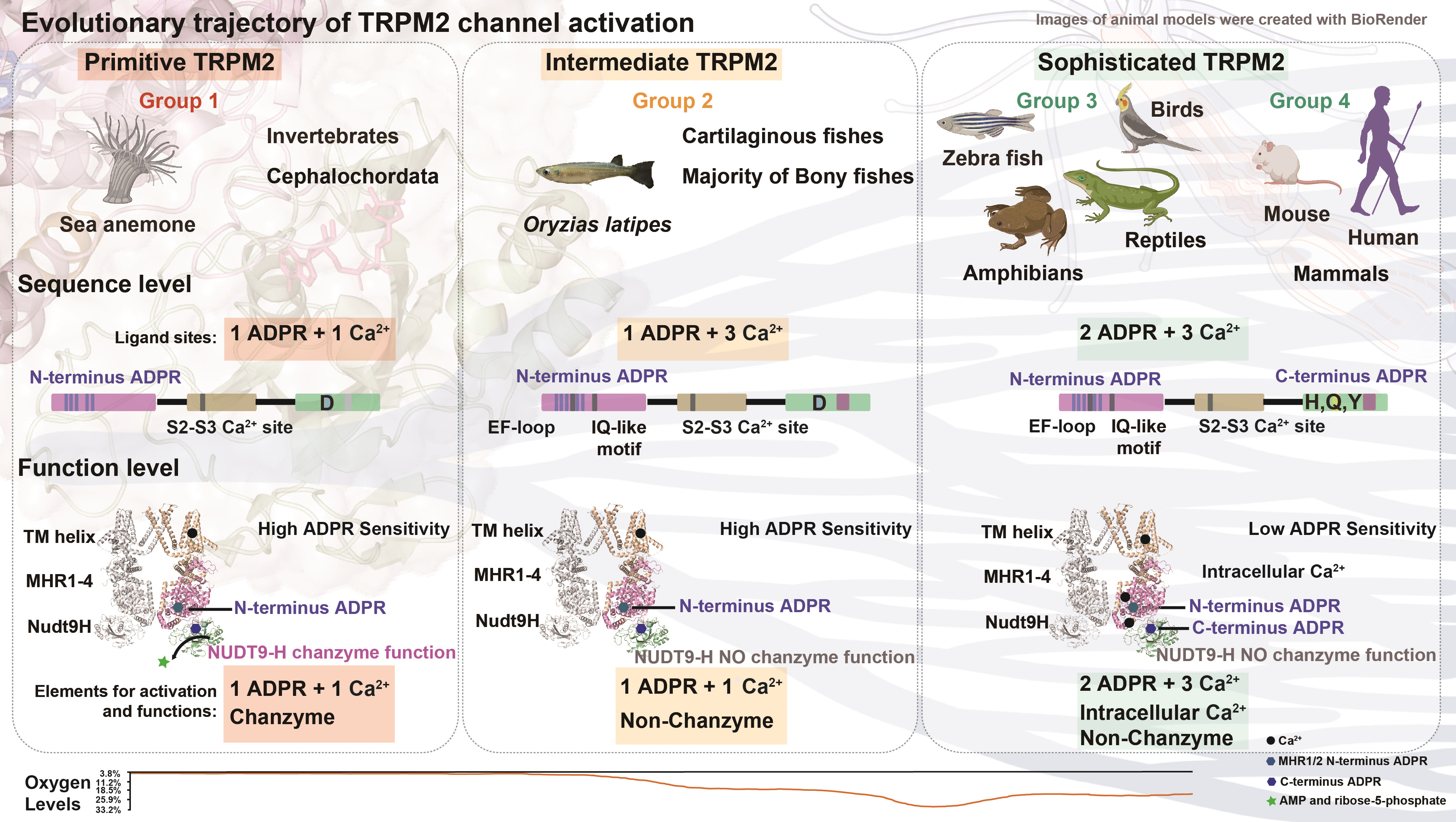
Ion channels, such as TRPM2, are ancient membrane proteins that regulate a multitude of physiological processes. TRPM2 plays a crucial role in oxidative stress-related diseases, neutrophil chemotaxis, insulin secretion, and body temperature regulation. The study reveals that the activation mode of TRPM2 has evolved from a simple mode in invertebrates to a more complex one in vertebrates.
The researchers identified three distinct stages in the evolution of TRPM2 channel activation. Initially, the C-terminal NUDT9-H domain evolved from an enzyme to a ligand binding site for activation, while the N-terminal MHR domain maintained a conserved ligand binding site. Secondly, the calcium gating pattern evolved from one Ca2+-binding site in sea anemones to three sites in humans. A novel gating mode in fish TRPM2 proteins was identified, representing an evolutionary intermediate. This discovery fills a missing link in the channel gating evolution.
The study suggests that environmental oxygen levels may have facilitated the development of a more precisely regulated activation mechanism for TRPM2 channels. This finding could provide insights into the evolution of other ion channels, receptors, or proteins, shedding light on how species adapt to their environments at the protein level.
The research employed a combination of molecular evolution techniques, mutagenesis, and patch-clamp recording to identify the evolutionary stages. The team also highlighted the importance of specific permissive changes at a certain position in the protein structure that occurred during the transition from water to land in vertebrates, indicating the origin of the NUDT9-H domain's dependence for channel activation by ADPR.
This groundbreaking research not only advances our understanding of ion channel evolution but also has implications for developing treatments targeting TRPM2-related diseases. The findings might lead to novel therapeutic strategies that leverage the intricate mechanisms of protein evolution.
See the article: Evolutionary trajectory of TRPM2 channel activation by adenosine diphosphate ribose and calcium.
Digital Object Identifier (DOI): 10.1016/j.scib.2024.04.052
Related Journal Name: Science Bulletin